Foncoo Pharmaceutical successfully introduced [Olanzhi] Olanzapine Tablets
Release Time
2022-06-21 15:32
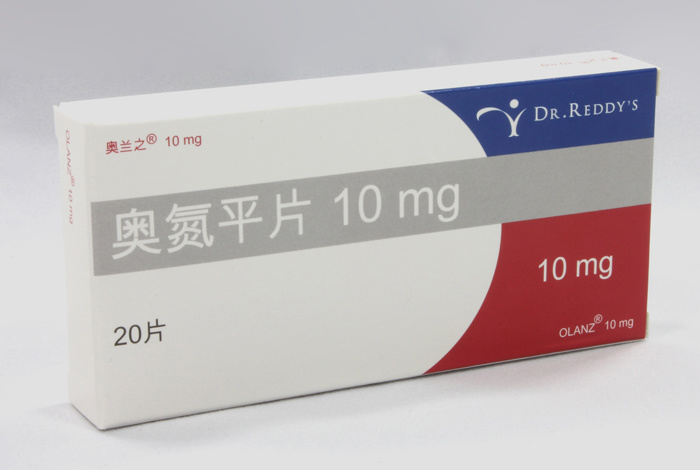
On September 23, 2013, Foncoo Pharmaceutical officially signed an agency agreement for psychoactive products with Dr. Reddy Laboratories Co., Ltd. of India, and successfully introduced [Olanzhi] Olanzapine Tablets (5mg and 10mg). Olanzapine Tablets is a first-line drug for the treatment of schizophrenia, with a large market capacity and excellent prospects.
[Olanzhi] Olanzapine Tablets are produced by Dr. Reddy Laboratories Ltd., the second largest pharmaceutical company in India. [Olanzhi] is an EU-certified product and has been marketed in the United States (the first imitation product), the United Kingdom, Switzerland, Australia, Italy, Brazil, and Singapore. It has obtained good clinical feedback in the treatment of schizophrenia and bipolar affective disorder. [Olanzhi] is an EU-quality product with Chinese prices, fully in line with modern pharmacoeconomics, and is the best olanzapine for cost-effectiveness analysis.
Previous page
Related News
Discover the Secrets of Jiuzhaigou and Find Serenity in Tianfu's Mountains and Waters
2025-04-24

Breaking through adversity, the remaining is king
2025-01-13

Gathering momentum for win-win, opening up a new future
2024-09-13

Join hands and embark on a new journey together
2023-11-08







